Download this Health Update as a printable PDF
Vaccine update
Multiple vaccines are in late stage clinical development in the U.S. and globally. Two vaccines that use messenger RNA technology targeting the COVID-19 virus spike protein are the leading candidates to reach the U.S. market first. Both manufacturers – Pfizer/BioNTech and Moderna – have publicly released the findings from their phase three studies and both indicate efficacy of approximately 95%. Both vaccines will require two doses spread 21-28 days apart.
Pfizer/BioNTech has just filed for Emergency Use Authorization (EUA) with the Food and Drug Administration (FDA) and others are expected to follow later in December or early January. This Clinical Update provides a summary of considerations as plan sponsors prepare for roll-out of a COVID-19 vaccine.
Distribution
Top officials from Operation Warp Speed, the government’s program to fast-track the development and delivery of COVID-19 vaccines, announced that they have allocated 6.4 million doses of COVID-19 vaccines to states based on population.
The allocation-by-population policy is a departure from earlier distribution plans, and it is a change from the independent vaccine advisory group for the Centers for Disease Control and Prevention (CDC). The CDC proposed allocation based on high-risk groups.
A Phased Approach to Vaccine Allocation for COVID-19
The states will now be responsible for creating vaccine distribution plans. Many governors have already modeled their statewide playbooks after the CDC guidance which is a phased approach as follows:
Phase 1a
- High-risk health care workers
- First responders
Phase 1b
- People of all ages with co-morbid and underlying conditions that put them at significantly higher risk
- Older adults living in congregate or overcrowded settings
Phase 2
- K-12 teachers and school staff and childcare workers
- Critical workers in high risk settings
- People in homeless shelters or group homes for people with disabilities
- People in prisons, jails, and detention facilities and the staff who work in these centers
- All older people and those with co-morbidities who were not included in Phase 1
Phase 3
- Young adults
- Children
- Workers in industries and occupations important to the functioning of society not included in Phase 1 or 2
Phase 4
- Everyone residing in the United States who did not have access to the vaccine in prior phases
Equity is crosscutting: In each population group, the vaccine should be prioritized for geographic areas identified through the CDC’s Social Vulnerability Index or another more specific index.
Source: https://www.nap.edu/catalog/25917/framework-for-equitable-allocation-of-covid-19-vaccine
Potential Timeline
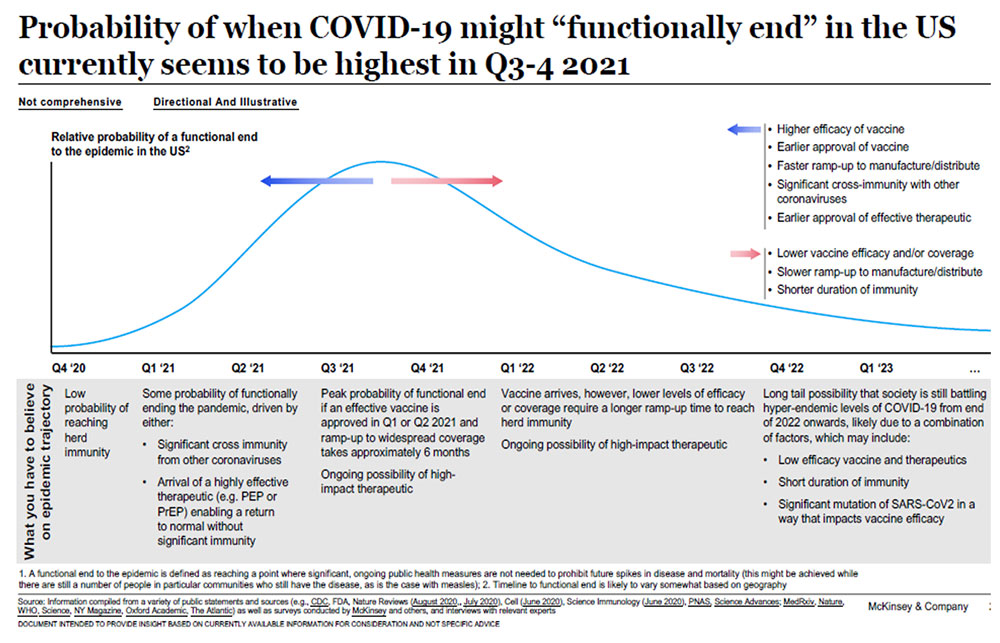
Many scientists are busy quantifying the timeline for the distribution of the vaccine and the “functional end” of the virus. McKinsey and Company published the following timeline model, preparing the United States for 2021 and beyond:
What Employer’s Need to Know
State public health departments will lead the distribution of the vaccine, and the U.S. Department of Health and Human Services will likely partner with national pharmacies like Walgreens and CVS to reach more people. The CDC is also looking at dispensing sites like healthcare systems, community vaccination providers, occupational health clinics, and employers.
Due to the special storage and handling requirements of these vaccines, distributing them across 3.5 million square miles is a logistical challenge. It may also take considerable effort to convince employees that the vaccine is safe and encourage them to receive it as soon as it is available. Although we are months away from the vaccine’s availability to the general public, it is essential that employers start preparing. Here’s where you can start:
Plan a strategy
- Create a COVID-19 vaccination committee to develop a strategy for your company.
- Distribute a survey and ask your employees if they would consider receiving a vaccine. If not, why? Overcoming these objections will be important to develop policy and return to work strategies.
- Explore how you will support employees in getting the vaccine:
- Paid time off
- Onsite administration
- Vouchers
- Incentives
Reach out
- Connect with your insurance carrier or pharmacy benefits management (PBM) team. Ask about their plans and potential resources in supporting Covid-19 vaccination administration.
- Are they coordinating administration efforts?
- What do they predict the administration fees to be?
- How are they planning to bill / pay these fees?
- Check with your local public health department, wellness vendors, and vaccination providers to see what resources will be available to support your vaccination strategy.
Provide support
- Encourage employees to receive the vaccine when it’s available. It’s not too early to start communicating.
- Communicate expectations with your employees.
- Plan to continue supporting virtual work and employee wellbeing and prevention messages, especially mental health, through 2021.
Updated Quarantine Guidelines
The CDC recently presented additional quarantine guidance at a White House coronavirus task force meeting. Previous guidance indicated the incubation period for the virus was thought to extend to 14 days. However, based on new studies / data, most individuals became infectious and developed symptoms between four and five days after exposure. While the CDC still recommends a 14-day quarantine period, due to this new information, shortened quarantine periods of seven and 10 days may be acceptable alternatives in specific situations. Specifics may be found at: https://www.cdc.gov/coronavirus/2019-ncov/index.html
Learn more
This health brief on clinical topics and innovations surrounding the SARS-coV-2 virus and COVID-19 disease was prepared by Buck’s Health Intelligence practice.
For more information, contact us at 866-355-6647 or talktous@buck.com.
| The information in this article is provided for general information purposes only and is not intended to address your requirements. While we will endeavor to keep the Information accurate, we cannot and do not guarantee the accuracy of the Information, and we accept no responsibility, and shall have no liability, for any loss or damage which may arise from using or relying on the Information. |
