Overview
A record number of confirmed cases, hospitalizations and deaths were reported this week as the virus continues to spread. Overall, the United States has more than 11.4 million confirmed COVID-19 cases since the pandemic began. Deaths have now exceeded 250,000.
Source: https://coronavirus.jhu.edu/us-map
Vaccine update
There are two vaccines developed, by Pfizer and BioNTech (“Pfizer”) and Moderna, that are preparing to request the Food and Drug Administration (FDA) emergency use authorization (EUA) within the upcoming days. Both vaccines report an initial efficacy rate in late stage trials of over 90%. Two other vaccine candidates, AstraZeneca-Oxford and Johnson & Johnson are in close pursuit. This is very encouraging, but there are some considerations as we move globally to distribute these vaccines equitably and safely.
Distribution
The Centers for Disease Control (CDC) and National Institutes for Health (NIH), as well as many states, have published COVID-19 vaccine distribution policies that may be complex due to the nature of the vaccine storage requirements. For example, Moderna’s vaccine remains stable for up to 30 days at 36 to 46 degrees Fahrenheit, the temperature of a standard home or medical refrigerator. It can be stored for up to six months at negative 4 degrees Fahrenheit. Pfizer’s candidate requires a storage temperature of minus 94 degrees Fahrenheit and requires special storage equipment and transportation. This vaccine can be kept in a standard home or medical refrigerator for up to five days. This could make either vaccine difficult to distribute in some areas of our country and globally.
There is speculation that there will be approximately 25 million doses available in the United Sates by year-end. This will be enough to vaccinate 12-13 million people (these vaccines require two doses). Then, it is estimated 20-50 million doses will be available each month thereafter, depending upon when each of the four companies receive EUA for their vaccines.
The CDC and the NIH plans would distribute the vaccine in four phases:
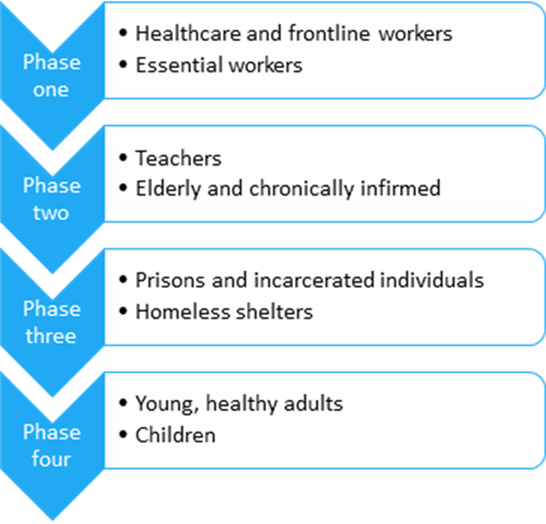
You may be already asking what that means for you individually and your organization? The Operation Warp Speed task force is working with medical distribution companies like McKesson to help disseminate the vaccine to hospitals and retail pharmacies, such as CVS and Walgreens. However, a proper storage freezer must be available when the vaccine arrives. Fortunately, many hospital systems pre-ordered sub-zero freezers in preparation for the vaccine months ago, and they are being delivered now.
Cost
The cost of the vaccine is also a concern for most Americans, particularly those who have lost jobs and healthcare benefits during the pandemic. The federal government is working to ensure that people can receive the vaccine at little to no cost. According to the CDC, the vaccine doses purchased with U.S. tax dollars will be provided to the American people at no cost. Although providers will be able to charge a fee to administer the shot, the fee will be reimbursed by the patient’s public or private insurance company or, for uninsured patients, by the Health Resources and Services Administration’s Provider Relief Fund.
Source: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
Reminder: Keep yourself and others safe from COVID-19 while at work and out in the community!
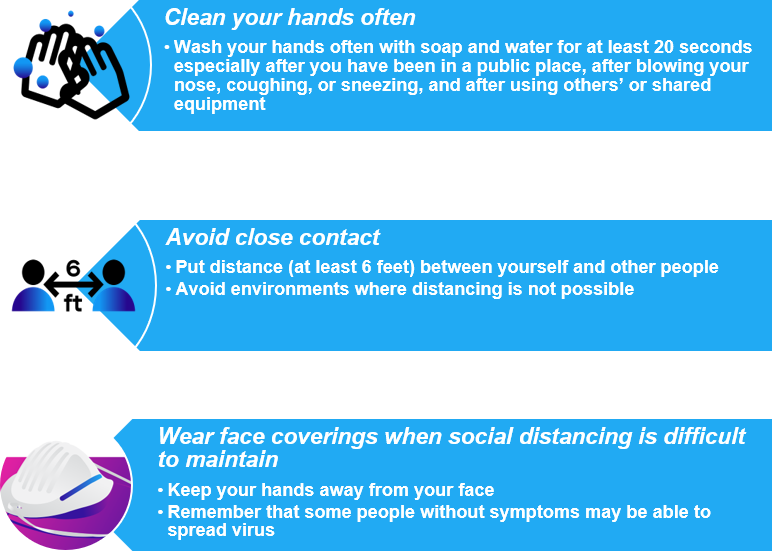
Source: https://www.cdc.gov/coronavirus/2019-ncov/downloads/community/Resuming-Business-Toolkit.pdf
Learn more
This health brief on clinical topics and innovations surrounding the SARS-coV-2 virus and COVID-19 disease was prepared by Buck’s Health Intelligence practice.
For more information, contact us at 866-355-6647 or talktous@buck.com.
| The information in this article is provided for general information purposes only and is not intended to address your requirements. While we will endeavor to keep the Information accurate, we cannot and do not guarantee the accuracy of the Information, and we accept no responsibility, and shall have no liability, for any loss or damage which may arise from using or relying on the Information. |
